HEK-Blue™ mNOD2
| Product | Unit size | Cat. code | Docs. | Qty. | Price | |
|---|---|---|---|---|---|---|
|
HEK-Blue™ mNOD2 cells Murine NOD2 expressing NF-κB–SEAP HEK293 reporter cells |
Show product |
3-7 x 10e6 cells |
hkb-mnod2
|
|
Murine NOD2 expressing HEK293 reporter cells
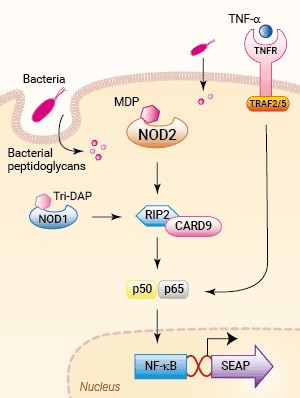
Signaling pathways in HEK-Blue™ mNOD2 cells
 InvivoGen also offers:
InvivoGen also offers:
• HEK-Blue™ NOD1 cells: NOD1 reporter cells
• NOD ligand collection: for activation studies
HEK-Blue™ mNOD2 cells were engineered from the human embryonic kidney HEK 293 cell line to assess the role of the Nucleotide-binding Oligomerization Domain-containing protein 2 (NOD2).
These cells stably express the murine (m) NOD2 gene and an NF-κB-inducible SEAP reporter gene. SEAP (secreted embryonic alkaline phosphatase) activity upon NOD2 stimulation can be readily determined by performing the assay in HEK-Blue™ Detection. This cell culture medium allows for real-time detection of SEAP. Alternatively, SEAP activity may be monitored using QUANTI-Blue™, a SEAP detection reagent.
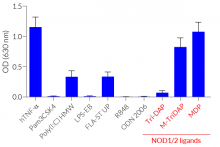
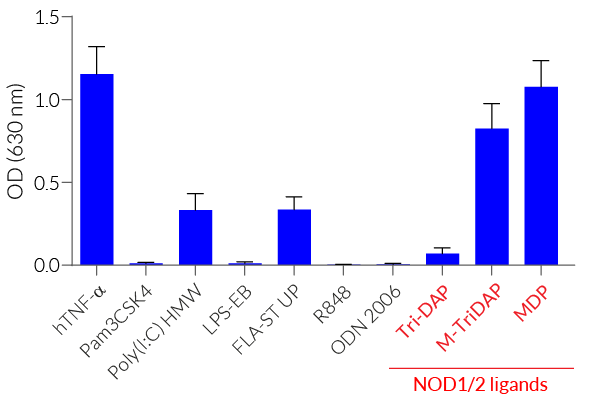
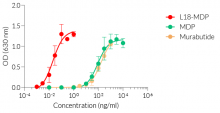
The overexpression of mNOD2 renders HEK-Blue™ mNOD2 cells to strongly respond to NOD2-specific ligands (see figures). As HEK293 cells express endogenous levels of NOD1, TLR3, and TLR5 [in-house data], HEK-Blue™ mNOD2 cells will respond to their cognate ligands such as Tri-DAP, Poly(I:C), and flagellin (see figure).
NOD1 and NOD2 are cytosolic pattern recognition receptors (PRRs), specialized to sense distinct motifs of peptidoglycan, an essential constituent of the bacterial cell wall. NOD2 recognizes the muramyl dipeptide (MDP) structure found in almost all bacteria, acting as a general sensor of bacterial invasion [1].
Key Features:
- Verified overexpression of murine NOD2
- Strong and stable response to NOD2-specific ligands
- Distinct monitoring of NF-κB by assessing the SEAP activities
Applications:
- Defining the distinct role of NOD2 in the NF-κB-dependent pathway
- Screening for NOD2-specific agonists or inhibitors in comparison with their parental cell line HEK-Blue™ Null2
References:
1. Correa RG, Milutinovic S, Reed JC,. 2012. Roles of NOD1 (NLRC1) and NOD2 (NLRC2) in innate immunity and inflammatory diseases. Biosci Rep. 32(6):597-608.
Back to the topSpecifications
Antibiotic resistance: Blasticidin, Zeocin®
Growth medium: DMEM, 4.5 g/l glucose, 2 mM L-glutamine, 10% (v/v) heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 μg/ml Normocin™
Quality Control:
- Murine NOD2 overexpression has been verified by RT-qPCR and functional assays.
- The stability for 20 passages, following thawing, has been verified.
- These cells are guaranteed mycoplasma-free.
Note: HEK 293 cells express endogenous NOD1, TLR3, and TLR5 levels.
The appropriate parental cell line for HEK-Blue™ mNOD2 is HEK-Blue™ Null2.
These cells are covered by a Limited Use License (See Terms and Conditions).
Back to the topContents
- 3-7 x 106 cells in a cryovial or shipping flask.
- 1 ml of Blasticidin (10 mg/ml)
- 1 ml of Zeocin® (100mg/ml)
- 1 ml of Normocin™ (50 mg/ml)
- 1 pouch of HEK-Blue™ Detection (cell culture medium for real-time detection of SEAP)
![]() Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
Shipped on dry ice (Europe, USA, Canada, and some areas in Asia)
FAQ Cell Lines
 Any questions about our cell lines ? Visit our frequently asked questions page
Any questions about our cell lines ? Visit our frequently asked questions page
Back to the top
Details
NOD1 and NOD2
The cytosolic NOD-Like Receptors (NLRs, also known as NODs or NALP) are Nucleotide-binding Oligomerization Domain containing receptors. To date, 22 NLRs have been identified in humans and constitute a major class of intracellular pattern recognition receptors (PRRs) [1].
The founding NLR-family members NOD1 (CARD4) and NOD2 (CARD15) recognize distinct motifs of peptidoglycan (PGN), an essential constituent of the bacterial cell wall. NOD1 senses the D-γ-glutamyl-meso-DAP dipeptide (iE-DAP), which is found in PGN of all Gram-negative and certain Gram-positive bacteria [1, 2] whereas NOD2 recognizes the muramyl dipeptide (MDP) structure found in almost all bacteria. Thus NOD2 acts as a general sensor of PGN and NOD1 is involved in the recognition of a specific subset of bacteria. Both iE-DAP and MDP must be delivered intracellularly either by bacteria that invade the cell or through other cellular uptake mechanisms. Ligand-bound NOD1 and NOD2 oligomerize and signal via the serine/threonine RIP2 kinase through CARD-CARD homophilic interactions [3]. Once activated, RIP2 mediates ubiquitination of NEMO/IKKγ leading to the activation of NF-κB and the production of inflammatory cytokines. Furthermore, poly-ubiquitinated RIP2 recruits TAK1, which leads to IKK complex activation and the activation of MAPKs [4].
Genetic mutations in NOD2 are associated with Crohn’s disease, a chronic inflammatory bowel disease [5]. In addition, numerous studies have recently revealed that NOD1 and NOD2 have a close relationship with a variety of cancers via controlling proliferation, altering immunosurveillance, and interacting with tissue bacteria, including intestinal commensal intestinal microflora. Moreover, additional research into the mechanisms of NOD1 and NOD2 in cancers would shed light on the innate immunity-cancer relationship and provide intriguing targets for immunotherapy [6].
References:
1. Chamaillard M. et al., 2003. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 4: 702-707.
2. Girardin S. et al., 2003. Nod1 detects a unique muropeptide from Gram-negative bacterial peptidoglycan. Science 300: 1584-1587.
3. Kobayash, K. et al., 2002. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416: 194-199.
4. Kobayashi K. et al., 2005. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307: 731-734.
5. Ogura Y. et al., 2001. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411: 603-606.
6. Wang D, 2022. NOD1 and NOD2 Are Potential Therapeutic Targets for Cancer Immunotherapy. Comput Intell Neurosci.;2022:2271788.